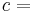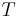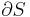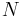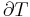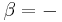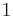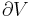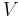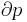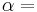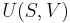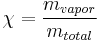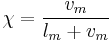Vapor quality
In thermodynamics, vapor quality is a quantitative description of the usefulness of a vapor to do mechanical work. The quality of a fluid is the percentage of mass that is vapor;[1] i.e. saturated vapor has a "quality" of 100%, and saturated liquid has a "quality" of 0%. For instance, in analysis of the Rankine cycle the quality of a multi-phase working fluid would be understood to imply this definition.
Vapor quality is an intensive property which can be used in conjunction with another independent intensive properties to specify the thermodynamic state of a thermodynamic system. It has no meaning for substances which are not saturated mixtures (i.e., compressed liquids or superheated vapors).
Quality 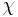 can be calculated by dividing the mass of the vapor by the mass of the total mixture:
can be calculated by dividing the mass of the vapor by the mass of the total mixture:
where 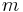 indicates mass.
indicates mass.
Another definition used by chemical engineers defines quality (q) of a fluid as the fraction that is saturated liquid.[2] By this definition, a saturated liquid has q = 1. A saturated vapor has q = 0.[3]
Calculation
The above expression for vapor quality can be expressed as:
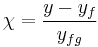 ,
,
where 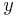 is equal to either specific enthalpy, specific entropy, specific volume or specific internal energy,
is equal to either specific enthalpy, specific entropy, specific volume or specific internal energy, 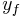 is the value of the specific property of the substance in the liquid state while under saturated conditions, and
is the value of the specific property of the substance in the liquid state while under saturated conditions, and 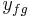 is the value of the specific property of the substance in the gas state minus that of the liquid state.
is the value of the specific property of the substance in the gas state minus that of the liquid state.
Another expression of the same concept is:
where 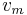 is the vapor mass and
is the vapor mass and 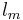 is the liquid mass.
is the liquid mass.
Steam quality
The genesis of the idea of vapor quality was derived from the origins of thermodynamics, where an important application was the steam engine. Low quality steam would contain a high moisture percentage and therefore damage components more easily. High quality steam would not corrode the steam engine. Steam engines use water vapor (steam) to drive pistons which create work. The quality of steam can be quantitatively described by steam quality (steam dryness), the proportion of saturated steam in a saturated water/steam mixture.[4] i.e., a steam quality of 0 indicates 100% water while a steam quality of 1 (or 100%) indicates 100% steam.
The quality of steam on which steam whistles are blown is variable and may affect frequency. Steam quality determines the velocity of sound, which declines with decreasing dryness due to the inertia of the liquid phase. Also, the specific volume of steam for a given temperature decreases with decreasing dryness.[5][6]
Steam quality is very useful in determining enthalpy of saturated water/steam mixtures since the enthalpy of steam (gaseous state) is many orders of magnitude higher than enthalpy of water (liquid state).
References
- ^ Cengel, Yunus A.; Boles, Michael A. (2002). Thermodynamics: an engineering approach. Boston: McGraw-Hill. pp. 79. ISBN 0-07-121688-X.
- ^ Wankat, Philip C. (1988). Equilibrium Staged Separations. Upper Saddle River, New Jersey: Prentice Hall. pp. 119–121. ISBN 0135009685.
- ^ Perry's Chemical Engineers' Handbook (7th Edition), p 13-29
- ^ http://www.mines.edu/Academic/chemeng/courses/dcgn210/Handouts/Steam%2520quality.pdf Steam quality
- ^ Soo, Shao L. (1989). Particulates and Continuum: A Multiphase Fluid Dynamics. CRC Press.
- ^ Menon, E. Sashi. (2005). Piping Calculations Manual. New York: McGraw-Hill.